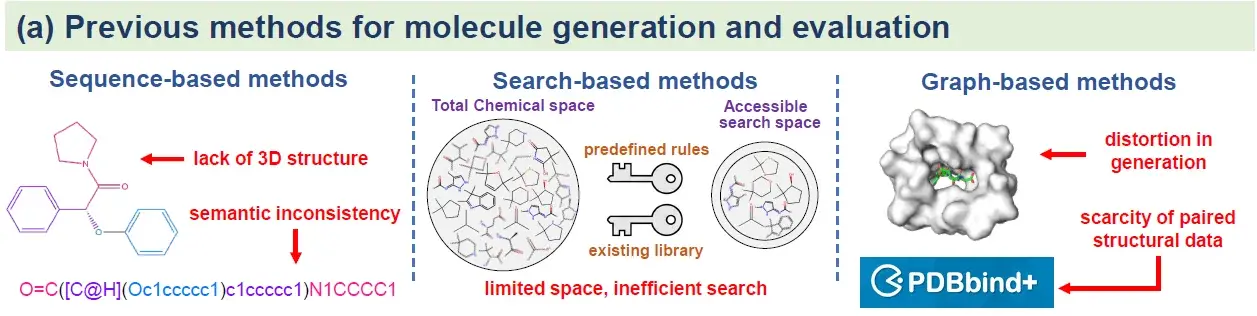
While recent molecular generation models provide powerful means to navigate chemical space and design novel compounds, they often reduce molecular design to over-atomized or over-symbolized representations. By prioritizing local interactions with binding-site residues through physics-based optimization, these approaches frequently neglect the semantic integrity of molecular functionality, undermining the plausibility of whole-molecule affinity. Moreover, the limited interpretability of current models remains a fundamental barrier: their black-box nature obscures the pathways of molecular optimization, leaving chemists unable to rationalize or trust the design outcomes, thereby constraining their broader adoption in drug discovery.
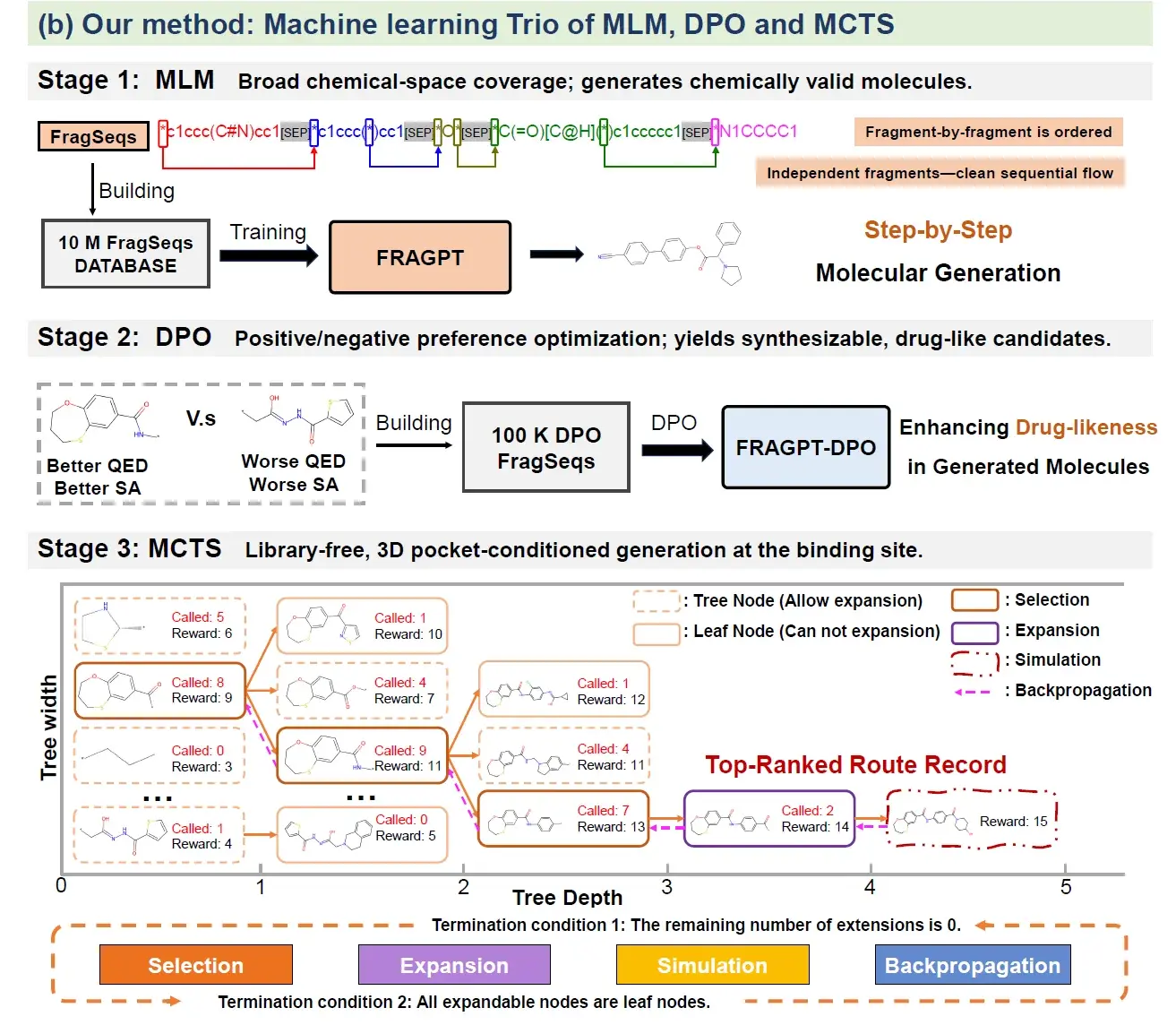
To jointly address generalization, plausibility, and interpretability, we propose Trio, a closed-loop paradigm that integrates a fragment-based MLM, reinforcement learning (RL), and Monte Carlo tree search (MCTS). At its core, a fragment-based MLM is trained on millions of SMILES strings to capture broad fragment sequence distributions and generate context-aware assemblies while circumventing the syntactic complexity of numeric junction identifiers and ring-index markers in SAFEGPT. To ensure drug-like plausibility, RL aligns the generative process with critical molecular properties such as QED and SA scores. Finally, the RL-aligned MLM then acts as the policy within MCTS, which explores fragment assembly trajectories in protein pockets using an upper confidence bound strategy to balance exploitation of promising structures against exploration of novel chemotypes, guided by affinity, pharmacokinetic, and SAR rewards. By combining fragment-level semantics, property-constrained optimization, and tree-based search, Trio achieves an interpretable and efficient molecular generation process that overcomes key limitations of prior approaches. Building upon this design, the backbone fragment–based MLM first demonstrates strong validity and novelty across both general de novo and constrained generation tasks. For the target-based molecular generation setting, Trio establishes a new performance benchmark, significantly outperforming state-of-the-art approaches through a superior balance of physicochemical properties. The model achieves robust gains over current baselines, improving drug-likeness by 11.10% and synthetic accessibility by 12.05%. Crucially, these enhancements are not achieved at the expense of potency; rather, Trio concurrently elevates predicted binding affinity (+7.85%) while notably expanding molecular diversity by fourfold. These results underscore the complementary strengths of fragment-informed MLMs, RL-driven property alignment, and MCTS-based strategic exploration, offering an effective and interpretable paradigm for targeted molecular design.